Mechanism of High-Temperature Cycling Degradation in NCM811 Lithium-Ion Batteries
With the rapid development of electric vehicles, energy storage systems, and high-performance electronics, lithium-ion batteries with high energy density have become the core focus of research and industrial application. Among them, NCM811 (LiNi₀.₈Co₀.₁Mn₀.₁O₂) — a nickel-rich layered oxide — stands out due to its high specific capacity and energy density.
However, NCM811-based batteries often face capacity fading, swelling, and shortened lifespan when operating under high-temperature conditions. This article analyzes the degradation mechanism of an NCM811/graphite pouch cell cycled at 45 °C, revealing the root causes behind its capacity decay.
1. Macroscopic Changes: The Warning Sign of Cell Swelling
After 500 charge–discharge cycles at 45 °C, the NCM811/graphite pouch cell shows noticeable swelling compared to the pristine cell. The cell thickness increases, suggesting that internal electrochemical and thermal reactions have occurred.
Two main factors contribute to this phenomenon:
Electrolyte decomposition and gas generation
Elevated temperatures accelerate the oxidative decomposition of the electrolyte, generating gases such as CO₂, H₂, and CH₄, which become trapped inside the pouch cell and cause swelling.Electrode material expansion
During cycling, both cathode and anode materials undergo repetitive volume changes as lithium ions intercalate and deintercalate, resulting in mechanical stress and expansion.
Upon disassembly, the pristine cell contains free-flowing electrolyte, whereas the cycled cell shows almost no liquid electrolyte remaining, indicating severe electrolyte consumption during high-temperature cycling.
This not only explains gas generation but also signifies intense parasitic reactions at the electrode/electrolyte interface, leading to the formation of resistive surface films and the loss of active lithium.
2. Cathode Structure and Morphology: Layered Framework Survives, Microcracks Emerge
X-ray diffraction (XRD) analysis reveals that both the fresh and cycled NCM811 cathodes maintain the typical α-NaFeO₂ layered structure (space group R3m). The main diffraction peaks remain, shifting slightly toward lower angles, implying lattice expansion along the c-axis. No new peaks corresponding to spinel or rock-salt phases are observed, meaning the bulk crystal structure remains intact even after high-temperature cycling.
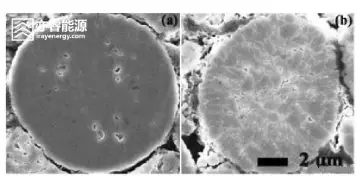
SEM images of the cathode before and after cycling
However, scanning electron microscopy (SEM) uncovers critical microstructural evolution:
Before cycling: Primary particles are tightly bound, grain boundaries are indistinct, and the overall structure is compact with few pores.
After cycling: Numerous cracks form along the grain boundaries of primary particles within the secondary aggregates, and in severe cases, particle fragmentation occurs.
This behavior is typical for high-nickel cathodes. During repeated charging and discharging, Ni³⁺/Ni⁴⁺ redox reactions induce significant lattice contraction and expansion. Due to crystallographic anisotropy, local stresses accumulate at grain boundaries, causing microcrack initiation and propagation.
Once cracks form, electrolyte penetrates deep into the secondary particles, triggering continuous side reactions and forming new surface films inside the material. This cyclic “crack–reaction–fragmentation” process progressively weakens the cathode integrity, ultimately leading to higher impedance and lower active material utilization.
3. Capacity Fading: Particle Fragmentation and Electronic Isolation
Electrochemical testing shows that the initial discharge capacity of the NCM811 cathode is 3.83 mAh, which decreases to 2.74 mAh after 500 cycles — a capacity retention of only 71.5%.
Although the bulk structure remains layered, microcracks and fragmentation result in several detrimental effects:
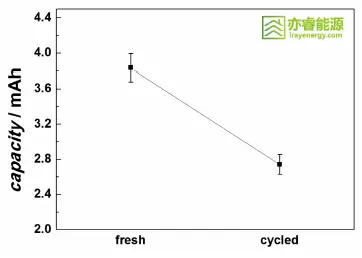
Coin cell capacity before and after cycling
Enhanced side reactions
Newly exposed surfaces react with electrolyte, forming inactive species such as NiO and consuming active lithium, which reduces the reversible capacity.Loss of electronic conductivity
Fragmented particles lose intimate contact with conductive additives and the current collector, increasing charge transfer resistance (Rct) and polarization during cycling.Active material detachment
Detached fragments no longer participate in electrochemical reactions, effectively reducing the amount of active material.
Thus, even without a structural phase transition, microstructural degradation and interfacial instability are the dominant causes of capacity fading under high-temperature cycling.
4. Mechanistic Summary of High-Temperature Degradation
Combining structural, morphological, and electrochemical evidence, the degradation mechanism of NCM811 under high-temperature conditions can be summarized as follows:
Severe electrolyte decomposition and consumption at elevated temperatures cause gas generation and interfacial thickening.
Anisotropic lattice expansion/contraction during lithium intercalation induces internal stress and crack formation.
Cracks promote electrolyte infiltration, accelerating interfacial side reactions and film growth.
Particle fracture and isolation lead to poor electronic pathways and increased impedance.
Overall, capacity fading and polarization rise as active sites and ionic/electronic conductivity are gradually lost.
In essence, the synergistic effects of internal stress-induced cracking and interfacial parasitic reactions are the root causes of performance degradation in NCM811 cathodes during high-temperature cycling.
5. Strategies to Improve High-Temperature Cycling Stability
To enhance the thermal stability and cycling life of NCM811 materials, several strategies can be implemented:
Optimization of synthesis conditions
Adjust sintering temperature, particle size, and elemental homogeneity to reduce structural anisotropy and internal stress.Surface coating
Apply protective coatings such as Al₂O₃, ZrO₂, or LiNbO₃ to isolate the cathode surface from direct electrolyte contact and suppress side reactions.Elemental doping
Incorporate dopants such as Mg, Al, or Ti to stabilize the lattice, inhibit Ni²⁺ migration to the Li layer, and mitigate phase transformation.Electrolyte optimization
Employ thermally stable solvents and additives (e.g., LiFSI, PES, TMSP) to enhance oxidative resistance and improve high-temperature compatibility.Structural design innovations
Use single-crystal particles or compactly packed secondary structures to minimize grain boundaries and suppress crack formation.
Through these material and system-level optimizations, the high-temperature cycling performance of NCM811 can be significantly improved, extending its service life in electric vehicles and energy storage systems.
6. Conclusion
Nickel-rich NCM811 cathode materials offer outstanding energy density and cost advantages, but their thermal instability and structural degradation remain major challenges.
This study demonstrates that, despite retaining a layered structure after high-temperature cycling, NCM811 suffers from crack formation, particle fragmentation, and interfacial side reactions, leading to polarization increase and capacity decay.
Future advancements will rely on synergistic material engineering, including particle design, surface/interface modification, and electrolyte optimization, to enhance the durability and reliability of high-nickel cathodes.
By addressing these degradation mechanisms, researchers and manufacturers can pave the way toward longer-lasting, high-energy lithium-ion batteries capable of stable operation even under elevated temperatures.