Three Main Causes of Lithium Plating in Lithium Batteries and Detection Methods
In the research, manufacturing, and failure analysis of lithium batteries, the term “lithium plating” frequently appears. Simply put, lithium plating occurs when lithium ions, which should normally intercalate into the anode active material, are abnormally reduced to metallic lithium and deposited on the anode surface or the separator. As the deposited lithium accumulates, it can eventually develop into lithium dendrites.
Common lithium dendrite morphologies include whisker-like, moss-like, tree-like, and spherical forms. Once formed, these dendrites not only cause capacity degradation but may also pierce the separator, leading to short circuits or even safety hazards.
So, why does lithium plating occur? From industry experience, it can mainly be analyzed from three aspects: usage patterns, design factors, and manufacturing processes.
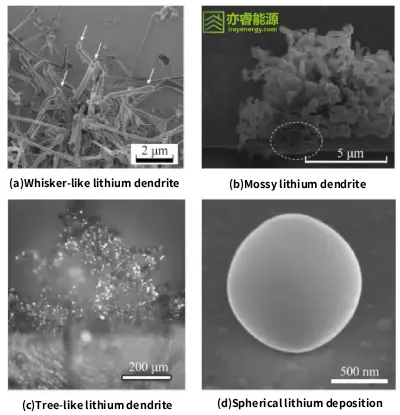
Lithium dendrite morphology diagram
1. Lithium Plating Caused by Improper Usage
Overcharging
When a battery is overcharged, the anode potential is forced below the lithium deposition potential (<0 V vs. Li/Li⁺). At this point, lithium ions can no longer intercalate into the anode and are instead deposited as metallic lithium on the anode surface. In other words, when the lithium released by the cathode exceeds the anode’s capacity to accommodate it, lithium plating occurs.
Charging at Low Temperatures
In low-temperature environments, electrolyte viscosity increases, slowing lithium-ion migration. Meanwhile, the anode’s kinetic performance deteriorates, hindering lithium intercalation and leading to surface accumulation and lithium plating. This is a critical risk for electric vehicles charging in winter conditions.
High-Rate Charging
When a battery is subjected to a current higher than its designed rate, internal polarization rises sharply, causing significant local potential fluctuations and greatly increasing the risk of lithium plating.
2. Lithium Plating Due to Design Issues
Improper Anode Material Selection
If the anode material has an excessively large specific surface area or uneven particle distribution, local current density may concentrate, which can induce lithium plating over prolonged operation.
Low CB Value
The CB value (also called the N/P ratio, i.e., the negative-to-positive excess factor) indicates the anode’s lithium storage capacity. If it is too low, the anode cannot fully accommodate the lithium released by the cathode, causing part of the lithium to deposit as metallic lithium, resulting in plating.
Electrolyte Mismatch
If the electrolyte formulation is unreasonable, its decomposition products may form a porous SEI (solid electrolyte interphase) on the anode surface, hindering lithium-ion diffusion and ultimately triggering lithium plating.

Lithium deposition caused by insufficient electrolyte injection diagram

Lithium deposition caused by excessive electrolyte loss diagram

Lithium deposition caused by high anode compaction and excessive electrolyte loss diagram

Lithium deposition caused by poor wetting in the electrode center diagram
3. Lithium Plating Caused by Manufacturing Processes
Even if design margins exist, uneven coating density may lead to local N/P ratios below 1, causing localized lithium plating. Additionally, untrimmed anode edges can create similar risks.
Excessive calendering can damage the anode structure, reducing lithium-ion intercalation ability and leading to plating.
Stacking Process
During stacking, poor coating, separator wrinkles, or trapped air bubbles can create internal structural defects in the cell, contributing to lithium plating.
Insufficient electrolyte wetting or air bubbles during filling can cause bubble-like lithium plating after full charge.
In soft-pack cells, if residual gases are not completely removed after formation and secondary sealing, they may also trigger lithium plating.
4. Lithium Plating Detection and Control
Some risk points in the manufacturing process (e.g., coating, calendering, stacking) can be mitigated through equipment adjustment and process optimization. However, issues during electrolyte wetting or formation degassing are often difficult to detect visually, and lithium plating during cycling cannot be monitored in real-time.
To address these industry challenges, iRay Energy has introduced ultrasonic detection technology in battery production to complement traditional blind spots:
During electrolyte wetting, ultrasonic imaging monitors wetting uniformity, preventing lithium plating caused by insufficient infiltration.
During formation degassing, ultrasonic technology verifies that gases are completely removed, reducing plating risks caused by residual gases.
This innovative detection approach effectively improves the identification of hidden defects, enhancing battery safety and reliability.
5. Conclusion
The root causes of lithium plating in lithium batteries may stem from improper user operation, design flaws, or manufacturing defects. Lithium plating not only accelerates capacity loss but can also evolve into hazardous lithium dendrites. Therefore, every stage—from design optimization and process control to proper usage—is crucial.
In the future, as advanced detection technologies such as ultrasonics become more widespread, the identification and control of lithium plating will become increasingly precise and efficient.