Will Batteries Always Swell at High Temperatures? — Improvement Countermeasures
Introduction
How can high-temperature performance be improved? To make it easier for readers to follow and understand, the editor from Iray Energy will break this question down into several perspectives.
1. Control of Water Content
Although moisture is only a minor impurity in lithium-ion batteries, it has a significant negative impact on high-temperature performance. The main effects are as follows: moisture catalyzes the decomposition of lithium salt LiPF6, thereby reducing battery capacity, while the generated HF further destroys the SEI film and causes gas generation.
In studying the thermal stability of electrolytes (EC:DMC = 1:1, LiPF6) under different water contents, we found that as the water content increases, the exothermic peak temperature of the electrolyte decreases (the higher the peak temperature, the more stable it is), and the total heat release also increases. The DSC curves of electrolytes with different water contents are shown below:
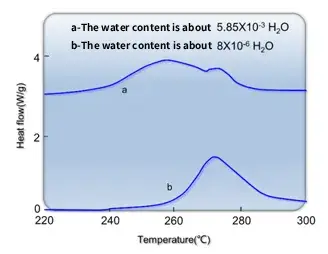
Therefore, in order to improve high-temperature performance, reducing the water content in both the electrodes and the electrolyte is a necessary measure. Of course, water content should not be excessively low either; an overall cell water content of around 150 ppm is considered an ideal value.
2. A Denser SEI Film
At high temperatures, the SEI film decomposes first (above 60 °C). When the temperature rises further or the cell is stored at high temperature for a long time, the SEI film will be severely damaged, exposing LiC6 to the electrolyte environment and triggering destructive reactions between the electrolyte and LiC6.
In this process, the SEI film plays a critical role both as a reaction participant and as a protector of the negative electrode. If a more stable and denser SEI film can be formed, the decomposition rate under high temperature can be reduced, and the negative electrode can be protected from side reactions with the electrolyte.
So how can a more stable and denser SEI film be formed? From a process perspective, the methods include: low-current formation, stepwise formation, and hot-press formation.
Take hot-press formation as an example. At higher formation temperatures, the reactions are more activated. While the SEI film keeps forming during the formation process, the metastable SEI components are continuously reorganized at high temperature into more stable SEI components. The final result is a thicker, denser, and more stable SEI film under high-temperature conditions. The impedance spectra of cells formed at different formation temperatures are shown below:
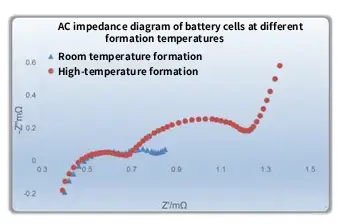
As seen in the figure, when the formation temperature increases, the AC impedance of the cell also increases, indicating that the SEI film formed is thicker and denser, thus delivering better high-temperature performance.
3. Larger-Particle Active Materials
The thermal stability of different cathode materials varies significantly, generally following the order:
LiFePO4 > LiMn2O4 > LiCoO2 > LiNi0.3Co0.3Mn0.3O2 > LiNi0.5Co0.2Mn0.3O2 > LiNiO2.
Therefore, in demanding high-temperature storage tests, if cathode materials with excessively high nickel content are used, special caution is required.
For carbon anode materials, the intercalation of lithium ions leads to an increase in interlayer spacing. For example, in commonly used carbon anodes MCMB and graphite, MCMB shows a smaller change in interlayer spacing after lithiation, which makes it more stable after charging and provides better high-temperature performance. A set of experimental comparison results is shown below:
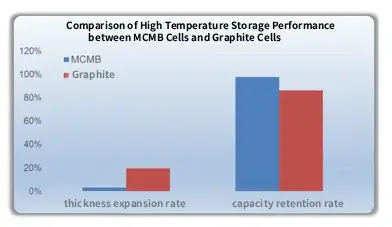
4. Use of Functional Additives
Additives for improving high-temperature performance can be categorized into three main types: adsorption-type additives, reaction-type additives, and film-forming additives.
Adsorption-type additives: Their primary role is to adsorb water and HF in the electrolyte, thereby suppressing their reactions with the SEI film and electrolyte solvents. For example, electrolytes with organosilanes containing Si–N bonds or acetals as additives can suppress the influence of water and HF on battery performance. Adding carbodiimides (R1N=C=NR2) to the electrolyte can also improve high-temperature performance, since the C=N double bond inhibits the formation of HF.
Reaction-type additives: These react with trace HF in the electrolyte to reduce its concentration and mitigate its destructive effects on the battery. Commonly used reaction-type additives include Al2O3, MgO, and BaO. For cathode materials, aluminum coating can also be applied to improve high-temperature performance, working via the same mechanism as adding Al2O3 into the electrolyte.
Film-forming additives: These function by forming a more stable and denser SEI film, enhancing the stability of the negative electrode under high temperatures and reducing side reactions with the electrolyte. Additives that can improve high-temperature performance include VC, PS, SO2, VEC, and others.
Summary of High-Temperature Improvement Methods
The main strategies for improving high-temperature performance include:
Controlling the overall cell water content to around 150 ppm,
Forming denser and more stable SEI films through high-temperature or low-current formation,
Using larger-particle active materials,
Choosing cathode and anode materials with inherently better thermal stability,
Utilizing functional additives that enhance high-temperature performance.
Conclusion
That concludes today’s discussion on high-temperature performance. Careful readers may have noticed that this series mainly focuses on improving high-temperature storage performance, while high-temperature safety aspects—such as thermal shock and extreme thermal runaway—have been touched upon only lightly. Since the topic is too broad to be fully covered in one series, the editor from Iray Energy will continue to explain these in the future. Hopefully, this article has given you some useful insights.








