How to Improve Battery Fast-Charging Performance
I. Introduction: Why Fast Charging Is a System Engineering Challenge
With the rapid popularization of electric vehicles (EVs) and smart devices, the demand for “rapid energy replenishment” has become increasingly urgent. Whether it’s an EV that users expect to charge fully in ten minutes, or a smartphone that supports “plug-and-go” charging, fast-charging capability has become a key indicator of battery technology advancement.
There are two primary approaches to achieving fast charging: high-voltage charging and high-current charging. The former allows more energy to be input within a short time but requires the system to have higher insulation strength and voltage endurance, which greatly increases cost. The latter speeds up charging by increasing current, but it often induces safety risks such as battery overheating, material degradation, and lithium dendrite formation. Therefore, fast charging is not merely a matter of “power stacking”; it is a complex multidimensional system technology involving battery design, electrolyte chemistry, charging strategy, and thermal management.
From a research perspective, fast-charging technology can be divided into two levels:
Macroscopic level: including charging pile power design, thermal management systems, pack balancing, and charging protocol optimization.
Microscopic level: focusing on internal material systems such as electrode architecture, electrolyte formulation, interfacial chemistry, and ion transport mechanisms.
Ultimately, the true determinant of whether a battery can achieve high-rate charging lies at the microscopic level — in the “genetic structure” of the cell itself. The core bottlenecks limiting fast-charging performance include:
Lithium-ion diffusion within electrode materials;
Lithium-ion migration through the electrolyte;
Lithium-ion desolvation and interfacial transfer processes.
Among these, the anode material plays a decisive role in avoiding lithium plating and polarization during high-rate charging. An ideal fast-charging anode should possess high electronic and ionic conductivity, stable structural and interfacial characteristics, and excellent electrochemical kinetics.
II. Improving the Fast-Charging Performance of Graphite
Graphite is the most mature and widely used anode material in commercial lithium-ion batteries due to its low cost, high stability, and excellent cycle life. However, its inherently slow lithium intercalation kinetics and low redox potential significantly limit its fast-charging capability. When charged at excessively high current densities, lithium metal can deposit on the graphite surface, leading to short circuits or thermal runaway.
To overcome this bottleneck, researchers have taken two major approaches:
Enhancing phase diffusion — improving lithium-ion transport within graphite particles and electrolyte;
Enhancing interfacial kinetics — optimizing lithium-ion desolvation and charge-transfer processes.
On this basis, a variety of graphite modification strategies have been developed, primarily including surface coating, structural optimization, and composite design.
III. Surface Coating: Building a “Fast-Charging Protection Layer”
Surface coating is one of the most effective and commonly used methods to enhance the fast-charging performance of graphite. By forming a thin layer of polymer, inorganic compound, or composite film on the graphite surface, multiple beneficial effects can be achieved:
Suppressing electrolyte decomposition and improving initial coulombic efficiency;
Reducing irreversible capacity loss;
Regulating interfacial reactions and accelerating lithium-ion desolvation;
Preventing co-intercalation of solvent molecules and protecting the layered graphite structure.
For example, turbostratic carbon coatings can significantly enhance the rate capability of graphite. Such carbon layers provide enlarged interlayer spacing and isotropic pathways, offering more active sites and diffusion channels for lithium ions. Similarly, graphite–hard carbon composites show outstanding fast-charging behavior, where an optimized ratio balances both energy and power density.
Moreover, certain inorganic oxides such as TiO₂ and Al₂O₃ serve as functional coating layers. TiO₂ coatings rich in oxygen vacancies promote interfacial ion transport, while amorphous Al₂O₃ enhances SEI stability, thus improving rate performance and cycle life. More promisingly, silicon–graphite composite materials combine high theoretical capacity with favorable lithium-ion kinetics, making them strong candidates for next-generation fast-charging batteries.
IV. Morphological and Structural Optimization: Building “Highways for Lithium Ions”
Beyond interface engineering, the morphology and structural design of graphite strongly influence fast-charging performance. Adjusting particle shape, porosity, and interlayer spacing can greatly improve lithium-ion diffusion rates and material utilization.
Particle size and distribution optimization:
Small graphite particles shorten diffusion pathways, facilitating rapid lithium transport; large particles, on the other hand, increase packing density and energy density. A mixed particle-size design, or “gradient distribution,” can balance both energy and power output.
Interlayer spacing regulation:
Chemical etching or thermal exfoliation can enlarge interlayer gaps and create microporous channels, reducing diffusion resistance and enhancing ion accessibility.
Defect and pore introduction:
Controlled structural defects can provide additional lithium storage sites and high-speed diffusion pathways. For instance, expanded graphite prepared via intercalation and exfoliation reactions exhibits a layered porous architecture, significantly improving rate capability.
Composite electrode design:
Combining graphite with carbon nanotubes or graphene prevents sheet stacking and establishes a continuous conductive network, enhancing both electronic and ionic transport.
Orientation control:
During electrode manufacturing, aligning graphite platelets toward the current direction reduces ionic transport resistance and further boosts fast-charging performance.
In essence, these structural optimizations act like constructing “express highways” for lithium ions, minimizing diffusion time and polarization during intercalation and deintercalation.
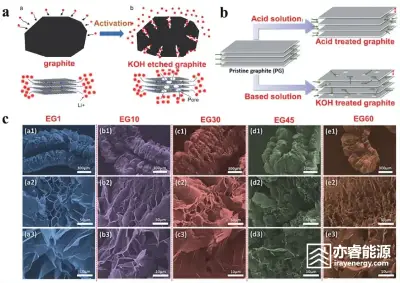
Figure 1
(a) Schematic diagram of the mechanism of graphite activation by KOH;
(b) Schematic diagram of the mechanism of acid-base activated graphite;
(c) SEM images of worm-like expanded graphite prepared under different conditions.
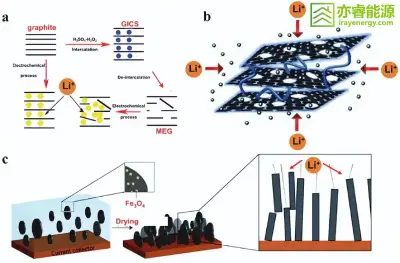
Figure 2
(a) Schematic illustration of the preparation of MEG and its mechanism for improving capacity;
(b) Diagram of lithium-ion intercalation in porous graphite nanosheets separated by carbon nanotubes;
(c) Schematic diagram showing how magnetically aligned graphite particles shorten the lithium-ion diffusion pathway.
V. Electrolyte Optimization: Creating an “Efficient Transport Medium”
The electrolyte is the most chemically active component in a lithium-ion battery. It determines ion transport, interfacial reactions, and SEI stability, all of which are crucial for fast charging. Electrolyte optimization primarily focuses on the following aspects:
Solvent system design:
Fast-charging electrolytes require low viscosity, high conductivity, and moderate desolvation energy. In traditional EC/EMC/DEC mixtures, linear carbonates improve ion mobility but may cause co-intercalation. Introducing low-desolvation-energy solvents (e.g., FEC, EMC) effectively reduces intercalation barriers.
Lithium salt selection:
Although LiPF₆ remains widely used, it easily decomposes into HF, which corrodes electrodes. Alternatives such as LiFSI or LiTFSI exhibit higher ionic conductivity and chemical stability. Mixed-salt systems (e.g., LiPF₆ + LiBOB) can synergistically form more stable SEI layers and enhance rate performance.
Functional additives:
Small amounts of VC, FEC, or phosphate-based additives preferentially reduce on graphite surfaces, forming dense and stable SEI layers that suppress solvent co-intercalation and improve safety and longevity.
Concentration control:
“Super-concentrated electrolytes” modify the solvation structure by increasing salt concentration, forming SEI layers rich in inorganic LiF, which provides high ionic conductivity and thermal stability. “Localized high-concentration electrolytes (LHCE)” introduce inert diluents to reduce viscosity and cost while maintaining stable interfacial chemistry.
Special ionic additives:
Incorporating cations such as Mn²⁺, Cu²⁺, or K⁺ can regulate lithium-ion solvation shells and accelerate interfacial charge transfer, greatly improving graphite’s fast-charging capability.
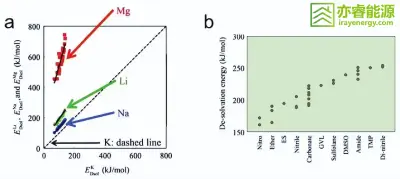
Figure 3
Schematic illustration of regulating the Li⁺ solvation structure.
In summary, by optimizing electrolyte composition and concentration, lithium-ion desolvation barriers can be reduced at the molecular level, improving interfacial kinetics and enabling faster and more stable charging.
VI. Optimizing Charging Strategies: Enabling “Smart Fast Charging”
In addition to material optimization, intelligent charging algorithms play a critical role in enhancing fast-charging performance. The conventional constant-current–constant-voltage (CC–CV) charging method is safe but limited at high rates due to polarization, dendrite formation, and reduced cycle life.
Researchers have proposed several advanced charging strategies:
Multi-stage constant current (MCC):
By gradually decreasing current density during charging, early-stage polarization and lithium plating can be mitigated.
Pulse charging (PC):
Periodic rest intervals allow concentration gradients to relax, reducing polarization and improving both efficiency and lifespan.
Dynamic current control:
By real-time monitoring of battery temperature, voltage, and internal resistance, the charging current is adaptively adjusted to maintain balanced ion and electron transport.
Temperature-assisted fast charging:
Moderate heating accelerates ion diffusion, though excessive temperature must be avoided. Recently, self-heating electrode designs (e.g., nickel-foil current collectors) have demonstrated uniform temperature rise and excellent fast-charging performance even at low temperatures.
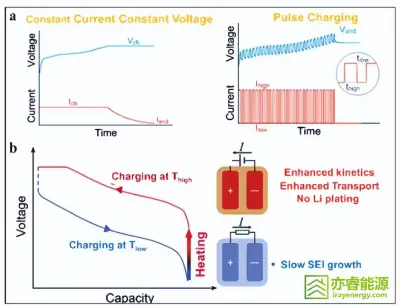
Figure 4
Schematic diagram of optimized fast-charging protocol.
A well-designed charging protocol can significantly accelerate charging without compromising safety or longevity, achieving a balance between performance and durability.
VII. Summary and Outlook: Toward the “10-Minute Full Charge” Future
Graphite remains the dominant anode material in lithium-ion batteries due to its maturity and cost advantages. However, to meet the ultra-fast-charging demands of EVs and large-scale energy storage, conventional graphite systems must continue evolving.
Effective strategies to enhance fast-charging performance include:
Building stable artificial SEI layers to improve interfacial ion conductivity;
Designing optimized morphology and structure to increase active sites and shorten diffusion paths;
Formulating advanced electrolytes to reduce desolvation energy barriers and suppress side reactions;
Developing intelligent charging protocols to balance charging rate and cycle life.
The next generation of fast-charging batteries will rely on synergistic innovation across materials, systems, and algorithms. With the integration of AI-driven battery management, solid-state electrolytes, and hybrid anode materials, achieving “10-minute full charge and 10-year lifespan” batteries is becoming an attainable goal.
Conclusion
The evolution of fast-charging technology represents a transition from “slow energy” to “instant energy” in modern energy storage systems. It not only accelerates the transformation of the automotive industry but also reshapes how humanity utilizes energy. Only through breakthroughs spanning material science, electrochemical mechanisms, and intelligent control systems can we truly enter an era of “charge anytime, go anywhere.”