How Do Lithium-Ion Batteries Work? Understand It in One Article
Lithium-ion batteries are almost everywhere—from smartphones and tablets to electric vehicles and energy storage systems, they make our lives more convenient. But do you know how this tiny battery works inside? In fact, its principle is both interesting and scientific.
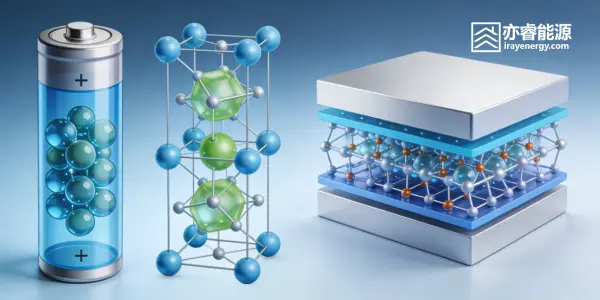
A lithium-ion battery mainly consists of a positive electrode, a negative electrode, an electrolyte, and a separator. The positive electrode is usually a lithium-containing intercalation compound, such as LiCoO₂, while the negative electrode often uses carbon materials like graphite. By “intercalation compound,” we mean a crystal structure that contains many vacancies, allowing lithium ions to freely move in and out like little travelers. These vacancies form channels, creating one-dimensional, two-dimensional, or even three-dimensional pathways for lithium ions to move smoothly during charging and discharging. A typical example is LiCoO₂ and graphite, both of which have a two-dimensional layered structure where lithium ions can freely “run back and forth” between layers.
When we charge the battery, lithium ions leave the positive electrode, pass through the electrolyte, and move to the negative electrode, embedding into the graphite lattice. At the same time, electrons flow through the external circuit to the negative electrode and “meet” the lithium ions, completing energy storage. The chemical reactions during charging can be expressed as:
Positive electrode: LiCoO₂ → Li₁₋ₓCoO₂ + xLi⁺ + xe⁻
Negative electrode: C + xLi⁺ + xe⁻ → LixC
When we use a device and the battery discharges, the process is exactly the opposite: lithium ions move back from the negative to the positive electrode, and electrons flow through the external circuit to provide power to the device. The overall chemical reaction can be expressed as:
LiCoO₂ + C → Li₁₋ₓCoO₂ + LixC
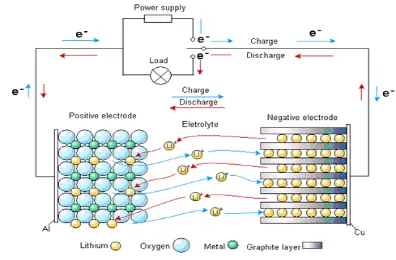
Working principle diagram of battery
Why can lithium-ion batteries work efficiently? The key lies in the intercalation compound structure of the electrodes. The design of vacancies and channels ensures that lithium ions move quickly during charging and discharging while keeping the electrode structure stable. This design gives the battery high energy density, long life, and good safety.
In daily use, to extend battery life, we can pay attention to a few small details: avoid overcharging and overdischarging, control charging temperature, and store the battery at 40%-60% charge if unused for a long time.
In summary, a lithium-ion battery works through the continuous transport of lithium ions and electrons between the positive and negative electrodes. The positive electrode provides lithium ions, the negative electrode “hosts” the lithium ions, and electrons complete energy transfer through the external circuit. This ingenious mechanism allows our smartphones, laptops, electric vehicles, and energy storage systems to operate reliably. Understanding how it works also helps us better appreciate technology and use our devices with confidence.