Will Batteries Always Swell at High Temperatures? — Mechanism of High-Temperature Swelling
Introduction
In the previous article on standards, the editor from Iray Energy shared the testing conditions for lithium-ion batteries under high temperature, and pointed out that the main performance degradations after high-temperature storage include capacity fade, thickness swelling, and increased internal resistance.
This naturally brings up the following question: What exactly happens inside a lithium-ion battery during high-temperature storage that causes these deteriorations?
The reactions occurring inside a lithium-ion battery during high-temperature storage can be categorized into three major types:
Reactions between the cathode material and the electrolyte
Reactions between the anode material and the electrolyte
Thermal decomposition of the electrolyte
Below, the editor will introduce them one by one.
1. Reactions between the Cathode Material and the Electrolyte
At high temperatures, fully charged lithium cobalt oxide (LiCoO₂) undergoes spontaneous thermal decomposition. The higher the temperature and charging voltage, the faster the decomposition proceeds. The reaction equations are as follows:
6Li0.5CoO2 → 3LiCoO2 + Co3O4 + O2↑
2Co3O4 → 6CoO + O2↑
The oxygen generated by the cathode decomposition further oxidizes the electrolyte solvents, producing CO2 and H2O. Example reactions include:
5O2 + 2C3H4O3 (EC) → 2CO2↑ + 2H2O
4O2 + C4H6O3 (PC) → 4CO2↑ + 3H2O
3O2 + C3H6O3 (DMC) → 3CO2↑ + 3H2O
So at what temperature does LiCoO₂ react with the electrolyte? Below is the DSC curve of LiCoO₂ immersed in electrolyte (EC:DMC:EMC = 1:1:1, 1 mol/L LiPF6):
From the DSC curve, it is clear that the reaction between LiCoO₂ and the electrolyte accelerates significantly above 150 ℃. Although reactions below 100 ℃ are not violent, long-term storage still leads to gradual decomposition.
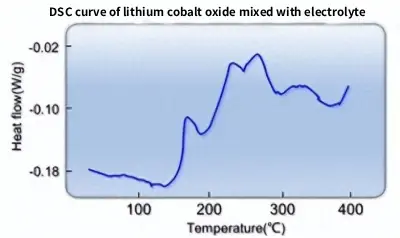
DSC curve of lithium cobalt oxide mixed with electrolyte
2. Reactions between the Anode Material and the Electrolyte
Although the fully charged anode material is not as oxidizing as the cathode, side reactions still occur as the temperature rises. The first side reaction is the thermal decomposition of the SEI (solid electrolyte interphase) film. Specifically, metastable compounds such as (CH2O-CO2Li)2 transform into more stable compounds such as Li2CO3. The reaction equations are:
(CH2O-CO2Li)2 → Li2CO3 + C2H4↑ + CO2↑ + 0.5O2↑
2Li+ (CH2O-CO2Li)2 → 2Li2CO3 + C2H4↑
The SEI decomposition temperature is relatively low, generally starting around 70–80 ℃, and the process is relatively slow. However, when the temperature rises above 160 ℃, the lithium-inserted carbon without SEI protection reacts violently with the electrolyte solvents:
2Li + C3H4O3 (EC) → Li2CO3 + C2H4↑
2Li + C4H6O3 (PC) → Li2CO3 + C3H6↑
2Li + C3H6O3 (DMC) → Li2CO3 + C2H6↑
Combining the above with the cathode’s high-temperature characteristics, we can see that when the temperature reaches ≥160 ℃, both the charged cathode and anode react violently with the electrolyte, generating large amounts of gas and releasing significant heat, which can ultimately trigger fire or even explosion. This is one of the reasons why the thermal runaway threshold of lithium-ion batteries is generally 130–150 ℃.
Since separators used in lithium-ion batteries typically have melting points in the range of 130–160 ℃, and the charged electrodes react violently with the electrolyte above 160 ℃, the thermal abuse test temperature is usually set in the critical range of 130–150 ℃. If set too high, safety cannot be ensured; if too low, the test cannot effectively differentiate performance.
3. Thermal Decomposition of the Electrolyte
At high temperatures, the electrolyte not only reacts intensively with active materials, but also undergoes its own decomposition reactions. These can be divided into two categories: intrinsic electrolyte decomposition and water-involved decomposition.
Examples of intrinsic decomposition reactions:
LiPF6 ⇌ LiF↓ + PF5
2C4H8O3 (EMC) → C5H10O3 (DEC) + C3H6O3 (DMC)
C3H6O3 (DMC) + PF5 → CH3OCOOPF4 + CH3F
CH3OCOOPF4 → PF3O + CO2↑ + CH4↑ + HF
C5H10O3 (DEC) + PF5 → C2H5OCOOPF4 + HF + C2H4↑
C2H5OCOOPF4 → PF3 + CO2↑ + C2H4↑ + HF
Thus, the decomposition products of lithium salts in the electrolyte react with linear carbonates to generate gases.
Water, as a major impurity in lithium-ion batteries, reacts with lithium salts to form HF, consuming active lithium. HF further damages the stable components of the SEI film and generates gas, while also reacting with solvents to produce additional gases:
PF5 + H2O → PF3O + 2HF
Li2CO3 + 2HF → H2CO3 + 2LiF↓
CH3OCOOPF4 + HF → PF4OH + CO2↑ + CH3F
C2H5OCOOPF4 + HF → PF4OH + CO2↑ + C2H5F
Summary of Causes for Performance Degradation After High-Temperature Storage
Capacity fade:
The reaction of the charged anode with electrolyte at high temperature, as well as the reactions of lithium salt decomposition products with solvents and water, consume active lithium, reducing reversible lithium ions and thus capacity. Cathode decomposition also damages its crystal structure, lowering the amount of lithium that can be inserted/extracted.Thickness swelling:
When storage conditions are insufficient to cause significant gas generation, a slight thickness increase (~1%) is mainly due to structural changes in electrode particles and SEI reformation. Once obvious gas generation occurs, the swelling is caused by CO2 and other oxidized products from cathode-electrolyte reactions, and hydrocarbons/olefins from anode-electrolyte reactions. If swelling decreases significantly after cooling, it indicates that electrolyte vaporization occurred during heating.Increased internal resistance:
Various reactions at high temperature generate solid by-products, which accumulate on the surfaces of electrode particles, thereby increasing the internal resistance of the cell.
Conclusion
The various side reactions occurring inside lithium-ion batteries at high temperatures are inevitable consequences of the materials used. However, for battery engineers, the real value lies in minimizing these adverse effects and enabling batteries to operate safely across a broader temperature window.
So, what can we do to improve high-temperature performance? That will be the focus of the next article from Iray Energy’s editor.