Battery Fast-Charging Technology | How to Improve Battery Fast-Charging Performance?
1.Introduction
Fast charging is mainly achieved through either high-voltage charging or high-current charging. High-voltage charging must be implemented under the premise of improving the vehicle’s insulation level and the robustness of electronic components (battery, motor, and electronic control systems), which significantly increases the production cost of electric vehicles. High-current charging also faces issues such as abnormal heat generation in the battery and accelerated aging of electrode materials. In addition, it requires compatibility between charging stations and the power grid. Therefore, fast-charging technology is a complex and highly integrated system.
For research purposes, fast-charging technology is divided into two categories: macro-level technology and micro-level technology. Macro-level technology focuses mainly on charging stations, thermal management, battery systems, and charging protocols, while micro-level technology concentrates on the battery’s internal ecosystem, such as electrode materials, electrolytes, and charge transfer interfaces. At present, macro-level technology is primarily dedicated to optimizing and maintaining fast-charging performance, while the decisive factor for fast-charging capability lies in the battery’s micro-level design.
Researchers summarize the micro-level limiting steps for fast-charging performance as follows:
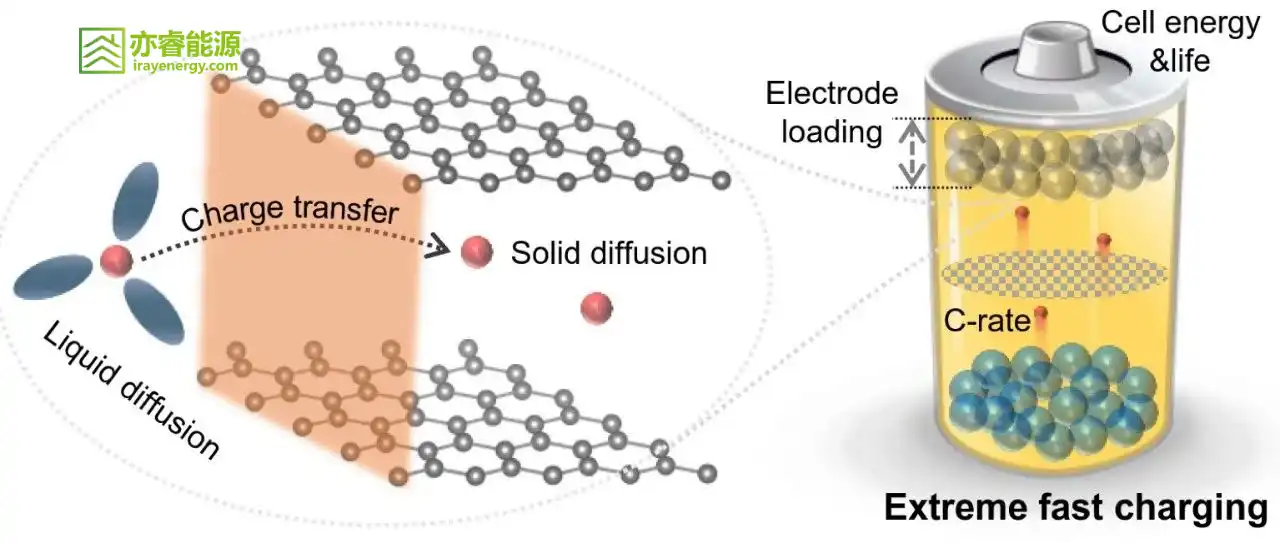
(1) Diffusion of lithium ions within electrode materials;
(2) Migration and diffusion of lithium ions in the electrolyte;
(3) Transfer of lithium ions at phase interfaces, including desolvation of electrolyte molecules and charge transfer at the interface.
The anode is key to achieving fast charging. For anode materials, the design strategies for fast charging are:
(1) Improve the ionic conductivity and electronic conductivity of materials to accelerate lithium-ion and charge transfer;
(2) Enhance the wettability of the electrolyte on the anode material surface to reduce interfacial transfer resistance;
(3) Suppress structural changes and volume expansion of the material under high-current conditions to improve cycle life;
(4) Prevent lithium plating on the anode surface to ensure safety performance.
2.Graphite Modification Strategies Based on Fast-Charging Requirements
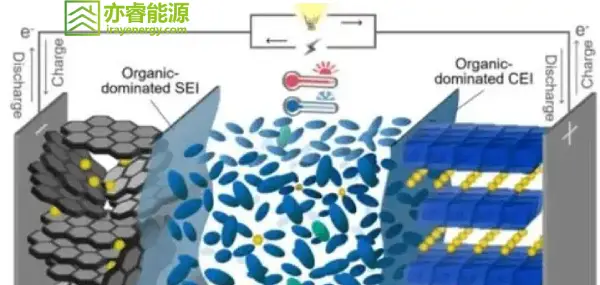
From a theoretical perspective, there are two main strategies to enhance the fast-charging capability of graphite: strengthening single-phase diffusion and enhancing interfacial kinetics. Strengthening single-phase diffusion refers to improving the diffusion ability of lithium ions either inside graphite particles or within the electrolyte. Enhancing interfacial kinetics refers to accelerating the desolvation of lithium ions and improving their migration capability across the solid electrolyte interphase (SEI) film.
In general, commonly used effective strategies include adjusting the solvation structure of lithium ions, regulating the structure at graphite boundaries, modifying bulk graphite materials, optimizing electrolyte composition, and optimizing charging protocols. By flexibly applying these strategies, researchers have developed a series of high-performance graphite anodes, providing valuable references for industrial production.
2.1 Surface Coating of Graphite
Recent research on graphite electrodes has focused on improving fast-charging performance through surface coating strategies. These strategies include pre-coating thin layers of polymers, inorganic compounds, and hybrid organic/inorganic layers on the surface of graphite particles. Such coatings can effectively suppress electrolyte decomposition, improve initial coulombic efficiency, and reduce irreversible capacity loss. At the same time, they can act as sieves for lithium ions and solvent molecules, accelerating the desolvation process of lithium ions and preventing the co-intercalation of solvent molecules, which could damage the graphite structure.
Scholars have conducted in-depth studies on the physicochemical properties of various coatings and their protection mechanisms for graphite electrodes. Among them, nanoscale turbostratic carbon coatings can significantly enhance the initial coulombic efficiency and specific capacity of graphite anodes. Thanks to their larger interlayer spacing and isotropy, they provide more active sites and fast transport channels for lithium-ion intercalation. In addition, graphite–hard carbon composites also exhibit excellent fast-charging performance, and battery performance can be systematically tuned by controlling the composition ratio.
Silicon–graphite composites represent another promising fast-charging material. Composites prepared via nickel-catalyzed hydrogenation and chemical vapor deposition not only possess enhanced lithium-ion kinetics but also feature a simple, efficient fabrication process with strong industrialization potential. Meanwhile, wet-chemical coating methods have also been successfully applied to deposit coatings such as oxygen-vacancy-rich TiO₂ and amorphous Al₂O₃ on graphite surfaces, significantly improving the rate performance of graphite.
2.2 Morphology and Structural Optimization
The fast-charging performance of graphite anodes can be significantly enhanced through various modification strategies. These strategies mainly include:
Microstructural design and structural improvement: By adjusting the particle size and particle size distribution of graphite, it is possible to optimize the active sites for lithium-ion intercalation and the diffusion pathways, thereby improving fast-charging capability. A reasonable mixed particle-size distribution can balance the high coulombic efficiency of large graphite particles with the high power density of small graphite particles. Spheronization treatment can reduce the anisotropy of graphite, increase active intercalation sites, and maintain high tap density.
Interlayer spacing regulation: Physicochemical methods can be used to adjust the interlayer spacing of graphite to widen the channels for lithium-ion intercalation and improve lithium-ion diffusion rates. For example, KOH etching, acid–alkali treatment, and thermal exfoliation can increase interlayer spacing and form porous structures, facilitating rapid lithium-ion migration.
Introduction of defects and porosity: Introducing defects and pores into graphite can provide more active sites, promoting lithium-ion storage and transport. Lightly expanded graphite is a typical example, prepared through intercalation reactions followed by thermally induced de-intercalation processes.
Composite electrode design: Combining graphite with other materials (such as carbon nanotubes) can create composite electrodes with special structures that facilitate lithium-ion transport within graphite layers. This design can prevent the re-stacking of graphite sheets and improve lithium-ion diffusion efficiency.
Regulating the stacking mode and physical orientation of graphite layers: By controlling the stacking arrangement and physical orientation of graphite layers, lithium-ion transport pathways can be further optimized. For instance, composite electrodes composed of thin graphite sheets with through-holes and carbon nanotubes can significantly accelerate interfacial charge transfer and enhance rate performance.
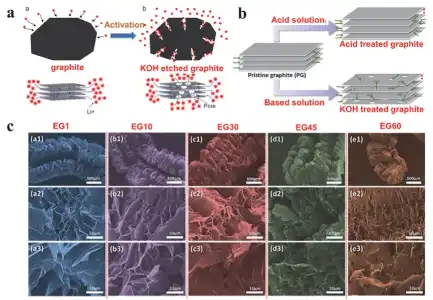
Figure 1 (a) Schematic diagram of the mechanism of KOH-activated graphite; (b) Schematic diagram of the mechanism of acid–base activated graphite; (c) SEM images of worm-like expanded graphite prepared under different conditions.
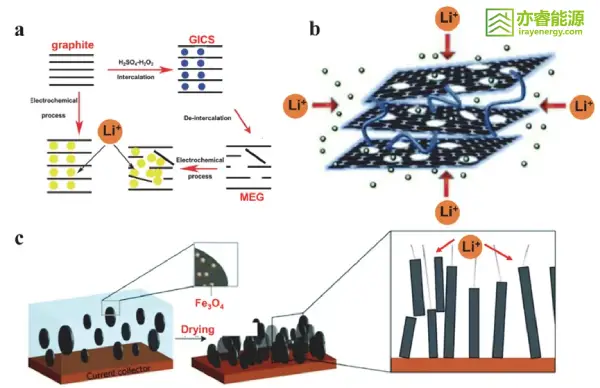
Figure 2 (a) Schematic diagram of MEG preparation and its mechanism for increasing capacity; (b) Schematic diagram of lithium-ion insertion in carbon nanotube-separated porous graphite nanosheets; (c) Schematic diagram of magnetically aligned graphite particles shortening lithium-ion diffusion paths.
3.Electrolyte Design
When discussing the factors influencing the fast-charging performance of graphite, the role of the electrolyte is particularly critical. The electrolyte not only affects the operating voltage, electrochemical performance, and working temperature range, but also directly impacts battery safety.
3.1 Choice of solvent
Solvation effect: The coordination between the solvent and lithium ions forms a solvation shell, which influences the lithium-ion intercalation process. The choice of solvent is crucial for the kinetics of desolvation.
Desolvation energy barrier: Different solvents have different desolvation energy barriers. Selecting solvents with lower energy barriers facilitates lithium-ion intercalation and improves fast-charging performance.
Co-intercalation issue: Solvent molecules may co-intercalate with lithium ions into the graphite interlayers, causing graphite exfoliation. Choosing solvents that can effectively suppress solvent co-intercalation is key to improving fast-charging performance.
3.2 Choice of lithium salt
Conductivity and environmental impact: Electrolytes containing lithium bis(fluorosulfonyl)imide (LiFSI) have high conductivity and lower fluorine content, making them a potential alternative to LiPF₆.
Synergistic effect: Using a mixture of lithium salts as the electrolyte, such as combining LiPF₆ with lithium bis(oxalato)borate (LiBOB), can form a more stable SEI film, improving the rate capability and cycling stability of graphite.
3.3 Role of functional electrolyte additives
Improving solvation structure: Certain functional electrolyte additives can modify the solvation structure, preventing solvent molecules from co-intercalating into the graphite anode.
Forming a stable SEI film: Additives can be reduced preferentially over the solvent, forming a stable SEI film that effectively prevents solvent co-intercalation and enhances the cycling stability of graphite under high current conditions.
3.4 Regulation of electrolyte concentration
Super-concentrated electrolytes: Increasing the lithium salt concentration in the electrolyte can significantly reduce the degree of solvation of lithium ions, leading to the formation of an SEI film dominated by inorganic components, thus improving the electrochemical performance of the graphite anode.
Use of diluents: Introducing inert diluents can reduce the viscosity and cost of the electrolyte while retaining the local coordination environment of the original concentrated electrolyte.
3.5 Influence of other additives
Metal cation additives: Metal cations such as Mn²⁺, Cu²⁺, Ni²⁺, and Ag⁺, when used as electrolyte additives, can regulate the solvation structure of lithium ions and significantly improve the fast-charging performance of graphite.
Specific salt additives: Potassium salts, cesium salts, and similar compounds can reduce the solvation binding energy between solvents and lithium ions, thereby reducing solvent co-intercalation.
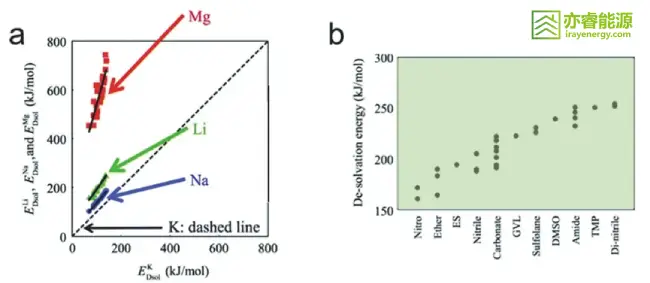
Figure 3 Regulation of Li⁺ solvation structure.
4.Optimizing Charging Strategies
The energy storage performance of lithium-ion batteries is highly dependent on the effective transport of lithium ions during charging and discharging. When lithium-ion transport is restricted—such as due to lithium plating on the graphite anode surface or aggravated cell polarization—electrochemical performance can decline significantly. To optimize lithium-ion transport, researchers have explored various modification methods, but these approaches are often tailored to specific materials and lack universality. In contrast, optimizing the charging protocol has become a widely applicable strategy, as it adjusts the electrochemical driving force during charging to match ion transport within the battery.
Optimized charging methods—such as multi-stage constant current charging, boost charging, dynamic pulse charging, and taper charging—aim to mitigate the lag in electrochemical reactions, synchronize electron transport in the circuit with ion transport, and thereby significantly reduce electrochemical polarization, unlocking the full energy storage potential of the materials. However, rapid charging often comes at the cost of reduced cycle life, so finding the right balance between charging time and battery lifespan is crucial.
Constant current–constant voltage (CC-CV) charging is a commonly used method to avoid lithium dendrite formation, but it has limitations under high-rate charging conditions, including dendrite formation, lithium-ion loss, additional SEI film growth, and increased interfacial polarization. Moreover, the prolonged CV stage often prevents a significant reduction in total charging time.
To address these issues, researchers have proposed pulse charging methods based on continuous variation of current rates, introducing short rest periods to improve relaxation, reduce concentration polarization, increase active material utilization, and extend battery lifespan. Additionally, temperature has a significant impact on the intrinsic kinetics of electrochemical reactions, meaning that the charging rate is also constrained by the battery’s operating temperature. Through controllable electrode structure designs—such as incorporating nickel foil as part of the current collector—electrodes can be heated rapidly and uniformly, suppressing lithium dendrite formation and achieving optimal fast-charging performance.
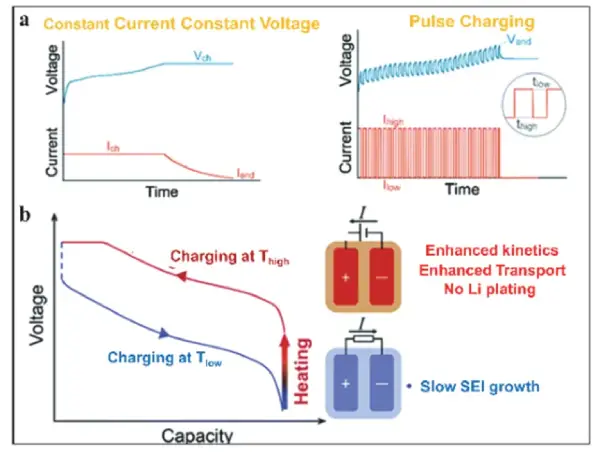
Figure 4 Optimization of fast-charging protocols.
5.Conclusion and Outlook
As the first commercially adopted anode material for lithium-ion batteries, graphite offers advantages such as high capacity, structural stability, and good electrical conductivity. More importantly, it is widely available, low-cost, and remains the most mainstream anode material, making it difficult to be completely replaced in the short term. With the widespread application of lithium-ion batteries in electric vehicles, fast-charging capability has become one of the most important performance indicators for graphite. However, due to slow lithium intercalation kinetics and an extremely low redox potential, the capacity, stability, and safety of graphite under high-rate charge/discharge cannot meet the demands of power batteries. Therefore, modifying graphite to improve its fast-charging performance has become a research focus in recent years. The current solutions to address graphite’s limitations in fast charging include:
(1) Constructing a stable artificial SEI film: By building a structurally stable, high-redox-potential, and ion-conductive organic/inorganic artificial SEI film on the graphite surface, it is possible to reduce anisotropy in lithium-ion transport within graphite, improve lithium-ion migration rates, lower polarization, and prevent lithium metal deposition on the graphite surface during high-rate cycling. In addition, an artificial SEI film can act as a “selective sieve” between lithium ions and solvent molecules, preventing co-intercalation of solvent molecules and the resulting damage to the graphite structure.
(2) Morphology and structural design: Improving the morphology and structure of graphite—such as designing porous structures—can increase the number of edge intercalation active sites, enhancing lithium-ion migration rates within graphite.
(3) Electrolyte optimization: By optimizing solvent selection, adjusting lithium salt type and concentration, and adding organic/inorganic additives, the solvation structure of lithium ions in the electrolyte can be effectively tuned. This reduces the desolvation energy barrier, forms a stable SEI film, and mitigates the detrimental effects of solvent co-intercalation on graphite stability.
(4) Optimizing charging strategies: By refining charging protocols, controlling charging current/voltage, and adjusting relaxation times, it is possible to reach the fast-charging rate limit while avoiding lithium dendrite formation, thereby achieving a balance between cycle life and charging speed.
All of these methods can effectively improve the capacity and stability of graphite under fast-charging conditions, providing valuable insights for enabling “refueling-like” charging experiences in electric vehicles.